![]()
A Beginner's Guide to the
Study of Plant Structure
Edward C. Yeung
Department of Biological Sciences
University of Calgary
Calgary, Alberta, Canada T2N 1N4
Tel: (403) 220-7186
yeung@acs.ucalgary.ca
Edward C. Yeung obtained his B.Sc. from the University of Guelph in 1972 and a Ph.D. in biology from Yale University in 1977. After spending one year as a postdoctoral fellow at the University of Ottawa, Dr. Yeung joined the Department of Biological Sciences, University of Calgary, where he is now a Professor. His primary research interests have been reproductive biology of higher plants, especially the structural and physiological aspects of embryo development.
© 1998 Edward C. Yeung
[ABLE's Copyright
Policy]
| Reprinted from: Yeung, E. C. 1998.
A beginner's guide to the study of plant
structure. Pages 125-141, in Tested studies for laboratory
teaching, Volume 19. (S. J. Karcher, Editor). Proceedings of the 19th
Workshop/Conference of the Association for Biology Laboratory Education
(ABLE), 365 pages.
Although the laboratory exercises in ABLE proceedings volumes have been tested and due consideration has been given to safety, individuals performing these exercises must assume all responsibilities for risk. The Association for Biology Laboratory Education (ABLE) disclaims any liability with regards to safety in connection with the use of the exercises in its proceedings volumes. |
Plant anatomy plays an important role in the understanding of plant biology. A realistic interpretation of morphology, physiology, and phylogeny must be based on a thorough knowledge of the structure of cells and tissues. Furthermore, the knowledge of plant structure is also essential to solve many important everyday problems such as the identification of unknowns, food contaminants, and forensic problems. The aim of this laboratory exercise is to introduce students to some useful techniques in the study of plant structure. At the same time, they will also learn the basic anatomical organization of plant organs, as well as cell and tissue characteristics.
The exercises require minimum costs to run and yet the methods will produce excellent results. This is primarily due to the fact that plant organs, especially stems and leaves, are firm enough that hand sections can be obtained readily. This, together with the use of simple staining schedules, allows the visualization of the structure using a light microscope. The exercise can be performed by students at all levels after having demonstrated the techniques to them. Students will need help initially in identifying cell and tissue types. Color photographs will be useful to serve as a guide for identification purposes. The techniques can be used to complement other laboratory exercises and can be used throughout the teaching term as required.
In this workshop, five areas will be covered:
A tray of supplies can be shared by two students:
The trays of materials, once prepared, can be used throughout the teaching term. The materials can be replenished as required.
Other supplies such as paper towels, filter papers, lens paper and lens cleaner for slides and microscope lens, and a first aid kit should be available in the laboratory.
Selected plant materials for examination.
Compound microscopes (1 per group of 2 students)
Fluorescent microscope
Plant anatomy is a basic core subject in the study of biology, especially plant biology. In the study of plant structure, it is important to recognize that there is a fundamental difference between plant and animal development. In plants, the environment plays a greater role in regulating development. As a result, plant cells are more adapted to changes. The internal structure of the same plant can be slightly different when grown in different environments. This is also reflected in their anatomy. Although distinct cell layers and tissues can be seen, different cell and tissue types do not occur as large homogeneous masses and no sharp demarcation exists as in animal organs. To complicate matters further, an apical to basal as well as a radial gradation of "age" exists within the plant body. As a result, differing structural characteristics exist. Therefore in order to learn about plant structures, it is important to take a hands-on approach. The purpose of the following exercises is to introduce some of the simple techniques that are useful in the study of plant structures. One will soon realize that one's own hand sections are better than prepared slides.
Most plant parts are too thick to be mounted intact and viewed with a microscope. In order to study the structural organization of the plant body, sections have to be made so that enough light can be transmitted through the specimen to resolve cell structures under the microscope. A free hand section is the simplest method of preparing specimens for microscopic viewing. This method allows one to examine the specimen in a few minutes. It is also suitable for a variety of plant materials, such as soft herbaceous stems and small woody twigs. The fixation of materials is generally not required for temporary preparations. "Patience, experience, and perhaps inherent skill are the chief requirements" for this technique (Berlyn and Miksche, 1976).
Procedures:
Obtain a new double edge razor blade. To minimize the risk of cutting oneself, cover one edge of the razor blade with masking tape. Rinse the blade with warm tap water to remove traces of grease from the surface of the blade if necessary.
Hold the plant material firmly. The material should be held against the side of the first finger of the left hand (or right hand) by means of the thumb. The first finger should be kept as straight as possible, while the thumb is kept well below the surface of the material out of the way of the razor edge (see Figure 9.1). Relax! It is not that easy to cut your own finger.
Flood the razor with water. This will reduce the friction during cutting as sections can float onto the surface of the blade. Take the razor blade in the right hand (or left hand) and place it on the first finger of the left hand (or right hand), more or less at a right angle to the specimen. See Figure 9.1.
Draw the razor across the top of the material in such a way as to give the material a drawing cut (about 45 in the horizontal direction). This results in less friction as the razor blade passes through the specimen. Cut several sections at a time. Sections will certainly vary in thickness. However, there will be usable ones among the "thick" sections!
Transfer sections to water, always using a brush, not a forceps or needle.
Select and transfer the thinnest sections (the more transparent ones) onto a glass slide and stain (see next section).
Note: For cross sections, special care should be taken during sectioning to see that the material is not cut obliquely. In our experience, as long as the sections are not obliquely sectioned, even "thick" sections are usable. During sectioning, a number of sections should be cut at the same time and one should not worry about the section thickness at this time. By slightly and progressively increasing the pressure with the razor blade on the first finger, and simultaneously exerting increasing pressure onto the specimen by the thumb, a number of sections can be cut without moving the material or the thumb. It is best to start cutting with the razor blade right at the surface of the specimen rather than against the side of the material. Since the root and stem usually have a radial symmetry, it is usually not necessary that a section should be complete, as long as it includes a portion of the tissues from the center to the outer edge of the specimen (O'Brien and McCully, 1981). Many additional free hand sectioning methods are available in the literature, please consult Cutler (1978), O'Brien and McCully (1981), and Purvis et al. (1966).
For delicate and hard to hold specimens such as thin leaves and tiny roots, additional support can be used to facilitate hand sectioning. The following methods will allow for the sectioning of thin leaves and small, soft specimens such as roots. As shown in Fig. 2A, tissue pieces can be inserted into a small piece of pith such as a carrot root. Once the tissue is firmly in place, the hand sectioning technique can be applied.
Longitudinal sections are also difficult to obtain by hand without supporting material as small stem and root pieces are difficult to hold with one's finger. However, by cutting a v-shaped notch into the pith support (Fig. 2B), it is possible to hold the tissue firmly for free hand sections.
Histological and histochemical staining techniques
Section staining is the most fascinating part in the preparation of specimens for microscopy. In general, most biological tissues have very little contrast, and cellular details are hard to discern with the ordinary light microscope. Stains can enhance and improve the visibility of the specimen. In addition, different stains have different affinities for various organelles and macromolecules. Therefore, the careful selection and utilization of stains can also suggest the chemical nature of the substances within the cell.
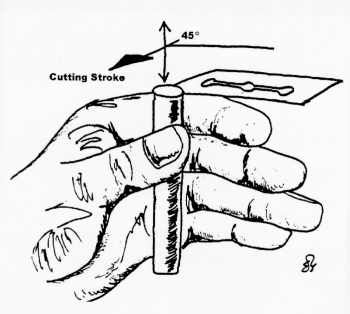
Figure 9.1. One method of holding a specimen for free hand sectioning.
The following staining procedures are used primarily for freehand sections only.
A general histological stain for free hand sections - Toluidine Blue O stain.
The stain, toluidine blue O (TBO), is an excellent stain for free hand sections. TBO has the advantage of being a polychromatic dye, i.e. it reacts with different chemical components of cells differently and results in a multi-colored specimen. The colors generated can provide information on the nature of the cell and its walls.
TBO is a cationic dye that binds to negatively charged groups. An aqueous solution of this dye is blue, but different colors are generated when the dye binds with different anionic groups in the cell. For example, a pinkish purple color will appear when the dye reacts with carboxylated polysaccharides such as pectic acid; green, greenish blue or bright blue with polyphenolic substances such as lignin and tannins; and purplish or greenish blue with nucleic acids (for details, see O'Brien et al., 1964).
Stain preparation: Dissolve 0.1 g of toluidine blue O in 100 ml of 0.1 M benzoate buffer, pH 4.4. (benzoic acid 0.25 g, sodium benzoate 0.29 g, water 200 ml). This buffer is recommended for histochemical purposes. If benzoate buffer is not available, for general use, tap water can be used as the solvent for TBO.
Staining procedures:
Note: Since water can evaporate from the slide over time, a 30% glycerol solution can be used instead of water. The sections will not dry out as fast as those in water.
Add only a small drop of mounting medium. Excess mounting medium around the cover glass should be removed by gently touching the edge of the cover glass with a filter paper. Be sure there is no mounting fluid on the surface of the cover glass. Be sure to place a cover glass over your preparation. Use only 1 cover glass!
Results: Pectin will be red or reddish purple; lignin, blue; other phenolic compounds, green to blue-green. Thin-walled parenchyma will be reddish purple; collenchyma, reddish purple; lignified elements such as tracheary elements and sclerenchyma will appear green to blue-green; sieve tubes and companion cells, purple; middle lamella, red to reddish purple; callose and starch, unstained (O'Brien et al., 1964).
Phloroglucinol-HCl test for lignin
Lignin is a common constituent in the secondary wall of plant cells; e.g., the walls of xylem elements and sclerenchyma tissue. The cinnamaldehyde end groups of lignin appear to react with phloroglucinol-HCl to give a red-violet color (Gahan, 1974). Although the reaction is not very sensitive, because of the ease of staining, this procedure is still often used as one of the tests for the presence of lignin in plant cell wall.
Stain preparation: There are various procedures to make up the staining solution but commonly it is prepared as a saturated solution of phloroglucinol in 20% hydrochloric acid. The hydrochloric acid used is about 2 N. Be sure to handle the solution with care. Wear gloves. Prepare this solution in the fume hood. First dissolve phloroglucinol (about 2.0 g) in 80 ml of 20% ethanol solution and then add 20 ml of concentrated HCl (12 N) to it.
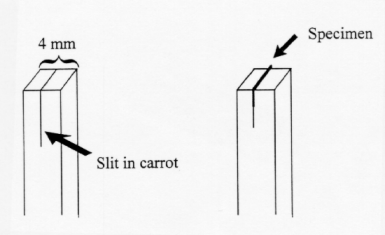
Figure 9.2A. A trimmed carrot block for holding thin specimen.
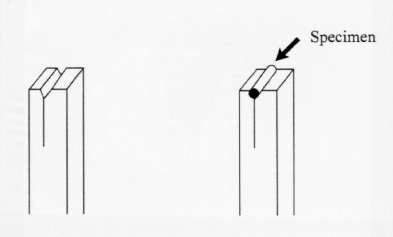
Figure 2B. A V-shaped notched is removed from the
carrot block
to accommodate a specimen for longitudinal sections.
Procedures:
Results: Lignified walls become red.
Starch: Iodine-Potassium-Iodide test (IKI)
The iodine-potassium iodide (IKI) stain is specific for starch. Apparently, the basis of the reaction is the accumulation of iodine in the center of the helical starch molecule. The length of the starch molecule determines the color of the reaction - the shorter the molecule, the more red the color; the longer the molecule, the more blue the color.
Stain Preparation: The IKI solution is prepared by first dissolving 2 g of KI in 100 ml of water, and adding 0.2 g of iodine into the KI solution. Prepare this solution ahead of time, as iodine takes some time to dissolve. Store the solution in a dark glass bottle and cap tightly. Exposure to light and air degrades the solution's usefulness. Iodine sublimates at room temperature. It is preferable to prepare the solution in a fume hood. The stain, once prepared, can be kept for several months or longer, as long as the bottle is tightly capped.
Procedure:
Results: Starches will give a blue-black color in a few minutes. Newly formed starch may appear red-purple.
Total Lipid - Sudan Dyes
The mechanism of staining is based on differential solubility. The Sudan dyes are more soluble in apolar solvents. As a result, they tend to dissolve more in structures such as the cuticle, lipid droplets, or suberin which are hydrophobic. Different methods are available in the preparation of Sudan dyes (see Jensen, 1962).
Staining solution: Staining solution is made by dissolving 0.7 g of the Sudan IV in 100 ml of propylene or ethylene glycol. Heat the solution to 100 C and stir it for several minutes. Filter the hot solution through Whatman No. 2 paper, cool, and filter again. Be careful when handling the hot solution.
Procedure:
Results: Fats, oils and waxes will stain red. The cuticle of leaves, suberized walls in the cork cells, and the casparian strip (suberin band) if present will stain red because of the lipidic nature of these structures.
A maceration method has been very useful in studying the features of intact cells. The following procedure is derived from a protocol developed by Brisson, Gardner and Peterson (Dr. Larry Peterson, Department of Botany, University of Guelph, Guelph, Ontario, Canada, personal communication). For a general discussion concerning various maceration techniques, see Gardner (1975).
In this maceration procedure, the middle lamella, which normally cements adjacent cells together, is dissolved by acid which allows the cells to separate from one another.
Maceration fluid preparation: The maceration fluid is prepared by combining 1 part of a 30% solution of hydrogen peroxide, 4 parts of distilled water, and 5 parts of glacial acetic acid. Be sure to use a clean bottle and prepare this solution in the fume hood. Avoid contact with the solution, wear gloves if necessary.
Procedures: Temporary preparations
Examples: The common polyhedral shape of parenchyma cells can be studied in gently macerated pith tissue. Place a small amount of macerated Coleus pith on a slide. Add a small drop of TBO. Stain for 1 min. In this exercise, do not wash the stain away from the macerated cell. Put a cover glass on the preparation and observe the shapes of the parenchyma cells.
Macerated woody samples can be used to study a number of xylem cell characteristics, such as the shape and size of tracheary elements, the types of secondary wall in tracheids and vessel members, perforation plates, and pits.
Fluorescence microscopy is becoming a popular method for the study of plant structure. Many unsaturated organic compounds can fluoresce when these compounds are excited and the absorbed energy is released instantaneously as light of a longer wavelength. A fluorescence microscope excites the compounds using a short wave-length (UV to blue region of the light spectrum, 350-480 nm) light source, such as the mercury vapor lamp. Through a combination of filters the UV and blue light are reflected, which allows the viewer to see only the fluorescent light of longer wavelength (usually beyond 500 nm of the light spectrum). The principle advantage of this method is that fluorescent compounds can be detected in very low concentration. Furthermore, many compounds in plants such as chlorophyll, lignin, suberin, cutin, and phenolic compounds can "autofluoresce" because of their intrinsic properties. Thus, simply using one's own free hand sections, one can identify some of these compounds without the need for staining. In addition, many specific techniques have been developed that allow one to stain for a number of macromolecules, such as callose in the phloem sieve plate and nucleic acids [see O'Brien and McCully (1981) and Ploem and Tanke (1987) for more details].
In this exercise, free hand sections will be examined for autofluoresence characteristics of plant cells and tissues. Since the specimen is illuminated from "above", i.e. epi-illumination, "thick" hand sections may also be used for this exercise.
Procedures:
Results: Autofluorescence characteristics of plant cell
Chlorophyll will appear red; lignin, blue. Cutin and suberin will be silvery white; phenolic compounds other than lignin will vary from green to blue. Note: The red chlorophyll fluorescence fades over time with continual exposure to UV excitation.
Free hand sections. Hand sections are not difficult to obtain, especially
transverse sections. In general, students should be asked to cut transverse
sections only because transverse sections are easier to obtain than longitudinal
sections. Furthermore, the internal structures are easier to identify in
transverse sections. The key for obtaining good transverse sections is that
the sections should be cut at right angles to the long axis of cells.
One would be surprised to find that many details can still be obtained even
with a "thick" section. It is important to note that some plant materials
work better for sectioning than others. The instructor should try to section
the material first before giving it to the students.
Different plant materials can be used for the study of plant structures.
One can obtain plant materials from grocery stores or floral shops. However,
if specific species are required, it is important to grow them ahead of time.
For example, four week old sunflower plants have many desirable features
to illustrate a variety of tissue types within the plant body.
Single edge razor blades will not give desirable sections. The knife edge
of a single edge razors has a wedge shape which will produce oblique sections.
Double edge razor blades are thinner with a smaller knife angle that will
give good quality transverse sections readily. To avoid cutting one’s
fingers, one side of the double edge blade can be covered using masking tape.
The razor blades can be taped before giving them to the students. If students
are going to use the free hand sectioning method throughout the entire term,
give each student a Petri dish with his/her name on it containing one or
two razor blades. A student can reuse a blade until it is dull before exchanging
it for a new one. Be sure to dispose of used blades properly. Furthermore,
it is essential that a first aid kit is available in the laboratory in the
event of an accident.
Solution preparation. All the solutions used in this chapter are easy to
prepare. They should be prepared ahead of time. All solutions, except for
the IKI solution, keep well at room temperature and will last for several
months. Since iodine sublimates slowly, the IKI bottles should be capped
tightly and may need to be replaced from time to time in order to maintain
the concentration of iodine as specified. The hydrochloric acid used in
conjunction with phloroglucinol is quite concentrated. Therefore, one should
take proper precaution in preparing and handling the strong acid.
Phloroglucinol-HCl test for lignin. For a better retention of the red color,
the sections need not be washed. They can be examined with the stain as the
mounting solution. If this method is used, take extreme care in handling
the slide.
Macerations. The hydrogen peroxide solution is a strong oxidant. Please read
the Material Safety Data Sheet for the proper storage and handling of hydrogen
peroxide solutions. Be careful not to contaminate the bottle. The maceration
solution should be prepared in the fume hood by adding hydrogen peroxide
and then glacial acetic acid to water. The solution must be capped tightly
for it to keep well. The timing for maceration depends on the tissue. For
soft tissue such as the parenchymatous pith of lettuce and tobacco, an overnight
treatment is sufficient. For woody tissue, several days are required and
fresh maceration solution may be needed as well. When tissue pieces turn
white, the maceration step is complete. After maceration, the vials should
be allowed to cool down prior to opening the screw cap. Wear gloves when
opening the vials to prevent getting the solution onto one's hand. The maceration
solution should be removed gently using a Pasteur pipette without disturbing
the tissues which are very fragile at this stage. The solution should be
exchanged several times with water to remove the acetic acid's smell. The
above steps should be carried out in the fume hood. The materials can be
stored for a long time. A protocol for preparing permanent preparations of
macerated material is detailed in Appendix C.
Fluorescence microscope. Care should be taken in the handling of the fluorescence microscope. It is essential that the instructor reads the operation manual and be familiar with the operation of the microscope. It is important to note that a fluorescence microscope should be left on once turned on. It is preferable to leave it on for at least an hour before turning it off. The lamp has to be completely cooled down before it can be turned on again. If not, there is a potential for malfunction, including the explosion of the mercury lamp. Mercury vapor is very poisonous. The mercury vapor lamp is only useful for a fixed number of hours. Beyond the recommended time, the light intensity becomes low and there is a danger of explosion. Thus, the total hours used must be noted and proper maintenance of the microscope is a must.
I wish to thank Dr. Chuck Curry and Ms. Nicole Ramesar-Fortner for their helpful comments on this manuscript.
Berlyn, G. P., and J. P. Miksche. 1976. Botanical microtechnique and cytochemistry. Iowa State University Press, Ames, Iowa, 326 pages. [ISBN 8138-0220-2]
Cutler, D. F. 1978. Applied plant anatomy. Longman, London, 103 pages. [ISBN 0-582-44128-5]
Esau, K. 1977. Anatomy of seed plants, second edition. Wiley & Sons, Inc., New York, 550 pages. [ISBN 0-471-24520-8]
Gahan, P. B. 1984. Plant histochemistry and cytochemistry - an introduction. Academic Press, London, 301 pages. [ISBN 0-12-273270-7]
Gardner, R. O. 1975. An overview of botanical clearing technique. Stain Technology, 50:99-105.
Jensen, W. A. 1962. Botanical histochemistry. Freeman, San Francisco, 408 pages.
Mahlberg, P. G. 1972. Laboratory program in plant anatomy. William C. Brown Company Publishers, Dubuque, Iowa, 342 pages. [ISBN 0-697-04555-2]
Mauseth, J. D. 1988. Plant Anatomy. Benjamin/Cummings Publishing Company, Menlo Park, California, 560 pages. [ISBN 0-8053-4570-1]
O'Brien, T. P., and M. E. McCully. 1981. The study of plant structure: Principles and selected methods. Termarcarphi Pty. Ltd., Melbourne, 344 pages. [ISBN 0-9594174-0-0]
O'Brien, T. P., N. Feder, and M. E. McCully. 1964. Polychromatic staining of Plant cell walls by toluidine blue O. Protoplasma, 59:367-373.
Ploem, J. S., and H. J. Tanke. 1987. Introduction to fluorescence microscopy. Oxford University Press, Oxford, 56 pages. [ISBN 0-19-856408-2]
Popham, R. A. 1966. Laboratory manual for plant anatomy. C. V. Mosby Company, St. Louis, 228 pages.
Purvis, M. J., D. C. Collier, and D. Walls. 1966. Laboratory techniques in Botany. Second edition. Butterworth Company Ltd., London, 439 pages.
Appendix A
Two dimensions vs. three dimensions
Conventional slide preparations only provide a two dimensional view of the object. However, biological specimens are three-dimensional objects. A two dimensional image cannot give a proper perspective of the internal construction of the specimen. Information based solely on two dimensional observations can be misleading. In order to obtain a three dimensional image of an object, serial sections and image reconstructions are needed. In most undergraduate courses, it is impossible to carry out these procedures. Even though three dimensional images cannot be demonstrated, the importance of the three dimensional concept should be conveyed to the student.
The following instructions are for a black agar block technique to illustrate the three dimensions of embedded specimens. While students will certainly understand the objective of this demonstration, it is more impressive to show them the agar block!
Note: As an alternative, a dark blue berry Jello™ powder can be used instead of the charcoal agar. A small amount of Knox™ gelatin must be added to the Jello™ powder to make it firm enough for slicing. The students can devour the demonstration after completing the exercise!
Appendix B
Useful plant materials for the study of plant structures
The following is a list of plant materials that can be used for class. The materials listed can be obtained easily from garden centers and commercial growers or the plants can be grown in a greenhouse or sun room.
Appendix C
Permanent macerated preparations
For teaching purposes, it may be convenient to have permanent preparations of macerated specimens. The following procedure details a protocol to make permanent slides.
Appendix D
Some anatomical features of the sunflower stem
In this appendix, the major anatomical features of the sunflower stem are illustrated. The purpose of the micrographs is to provide a guide for identification purposes. For more details about the internal stem anatomy, please consult plant anatomy texts such as Esau (1977) and Mauseth (1988).
The epidermis is the outer protective covering for the plant organ (Fig. 9.3A). In the sunflower stem, besides normal epidermal cells, both secretory and non-secretory types of trichomes are present. The secretory trichomes are short while the non-secretory trichomes are long with a pointed tip.
Underneath the epidermis is the cortex of the stem. Two major tissues can be found in the cortex. The collenchyma is located immediately beneath the epidermis and the parenchyma is located near the vascular bundles. Several types of collenchyma cells can be found (Fig. 9.3A). Usually, the lamellar collenchyma cells are located underneath the epidermis followed by the angular and lacunar collenchyma cells (Fig. 9.3A). Depending on the age of the plants, all three types of collenchyma cells may not be present. The parenchyma cells of the cortex are isodiametric in shape. Chloroplasts can be found within these parenchyma cells. The innermost layer of the cortical parenchyma cells tend to be larger than adjoining cells and the plastids containing starch grains can readily be detected using the IKI stain. This layer of cells in the sunflower stem is known as the starch sheath. Both the collenchyma and the parenchyma cells stain purple with TBO indicating the primary nature of their cell wall. Internal secretory canals can also be found in the cortex of the stem.
The vascular tissues are grouped in the form of bundles (Fig. 9.3B). The phloem is located towards the epidermis while the xylem tissue is located near the pith (Fig. 9.3B). The phloem is a complex tissue. Phloem fibers form a protected cap just outside of the conducting elements of phloem. At maturity, an intense blue color can be seen when phloem fibers are stained with TBO. However, for young fiber cells, the cells give a purple color reaction with TBO as lignin has not yet deposited in the cell wall. The primary phloem contains three major types of cells, i.e. phloem parenchyma cells, sieve tube members, and companion cells (Fig. 9.3C). The companion cells can be identified readily as they are small in size and densely stained. In the case of sunflowers, 1 or 2 companion cells are found to associate with a sieve tube in transverse section. The sieve elements are angularly shaped, especially when sectioned near a sieve plate (Fig. 9.3C). The cell walls give a more intense purple color. The phloem parenchyma cells are large and have a more irregular shape (Fig. 9.3C). Because of the contrasting features among these three cell types, they can be readily identified.
At the primary state of growth, the procambium serves to separate the primary phloem and primary xylem. In the mature part of the stem, i.e. near its base, the procambium will differentiate and give rise directly to the vascular cambium (Fig. 9.3C). In general, the procambial cells as well as the vascular cambial cells have a uniform arrangement; several layers of rectangularly-shaped cells can be seen separating the phloem and xylem.
The xylem of the sunflower stem consists of vessel elements and parenchyma cells. The vessel elements appear as large pores in a transverse section (Fig. 9.3D). Vessels of different sizes can be found in the stem. The vessel elements that are formed first, i.e. the protoxylem, tend to have a smaller diameter as they are being stretched during stem elongation. Furthermore, due to the rapid elongation process, the protoxylem elements can be torn to create a protoxylem lacunae (Fig. 9.3D). The protoxylem is located near the pith. For those vessel elements that mature late, i.e. the metaxylem vessel elements, the diameter of the cells tend to be larger than the protoxylem elements as they have more time to expand before maturation. One of the characteristics of the vessel elements is that the secondary wall containing lignin is present; therefore, mature vessel elements will stain blue with TBO. The cells surrounding the vessel elements are xylem parenchyma cells (Fig. 9.3D). At the early state of growth, the cell wall will react with TBO and give a purple color indicating it is still primary in nature. However, in older stems, the xylem parenchyma cells become lignified to provide additional support for the xylem tissue. At this time, the xylem parenchyma cells will give an intense blue color indicating the presence of lignin.
In the center of the stem are the pith parenchyma cells. These cells are large and stain purple with the TBO stain. Due to the rapid elongation and expansion of the stem, the pith can be torn to create a cavity.
Appendix E
Suppliers
All chemicals mentioned in this chapter can be purchased from the Sigma Chemical Company, P.O. Box 14598, St. Louis, MO 63178-9916, U.S.A., 1-800-521-8956.
Other general laboratory supplies such as brushes, dropper's bottles, slides and coverglasses can be obtained from a number of scientific supplies companies such as Fisher Scientific, VWR Scientific, Cole-Palmer, etc.
Double edge razor blades can be obtained from Electron Microscopy Science, 321 Morris Road, Box 251, Fort Washington, PA 19034, U.S.A., 1-800-523-5847.
The mounting medium Cytoseal 60 can be obtained from Stephens Scientific, Division of Cornwell Corporation, Riverdale, NJ 07457-1710. This product can be ordered through VWR Scientific, 1-800-932-5000. The mounting medium Permount from Fisher Scientific is an excellent alternative.
Figure 9.3 is on the adjacent page.
Figure 9.3. illustrates the major anatomical features of a sunflower stem. Transverse sections were obtained using the free hand sectioning procedure and stained with TBO.
Fig. 9.3A. The epidermis (E) forms the outermost protective covering of the stem. Different types of collenchyma cells, i.e. lamellar (L), angular (arrowhead), and lacunar (arrow) collenchyma cells can be found underneath the epidermis. The inner region of the cortex is occupied by parenchyma cells. Scale bar = 20 mm.
Fig. 9.3B. A low magnification micrograph to show the essential features of a vascular bundle. A fiber cap (F) is located outside of the phloem (Ph) elements. The xylem (X) elements are located near to the pith. The procambium is sandwiched between the phloem and xylem. In this section, the procambium begins to differentiate into the vascular cambium (*). Scale bar = 40 mm.
Fig. 9.3C. This high magnification micrograph illustrates some features of the primary phloem and the vascular cambium. The companion cells (arrowheads) are small and densely stained. The sieve tube elements (arrow) are larger than the companion cells in the transverse section. In the metaphloem, sieve tube elements are always found in association with 1 or 2 companion cells in transverse sections. The phloem parenchyma cells (*) are the largest type of cells in the primary phloem. The shape and size of the parenchyma cells vary. The cells in the developing vascular cambium (VC) are rectangular in shape and have an uniform arrangement. Scale bar = 20 mm.
Fig. 9.3D. The xylem vessel elements appear as large circular pores (V). The protoxylem lies next to the pith. In this transverse section, the protoxylem vessel elements have been torn to create protoxylem lacunae (*). The metaxylem vessel elements appears as rows or files. The vessel elements are surrounded by xylem parenchyma cells. Scale bar = 20 mm.
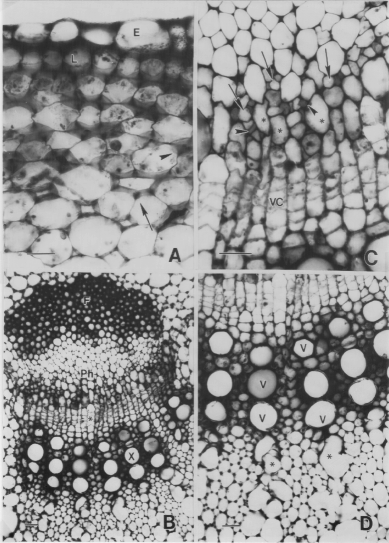
Figure 9.3. Anatomical features of a sunflower stem.

All contents copyright © 2000. Association for Biology Laboratory Education. All rights reserved.